TREE LIFE
February 2021
We are once again confined to barracks by Covid 19 and all Tree Society activities have been cancelled.
We really hope that the various invitations can be postponed until we are facing better times.
Of pawnbrokers and potpourri — Tree of the month — Excoecaria bussei

Baobab trunk. Photo: Ryan Truscott
Back in November, the Tree Society paid a visit to the National Botanic Garden — the first in eight months. It was the height of the drought. The garden was so dry even the trunks of the baobabs were visibly shrunken.
Many of the trees in the section we explored were bare. It was a relief to come across plants that still had flowers or leaves.
One of these — the Pawnbroker tree, Excoecaria bussei had both.

Pawnbroker flowers. Photo: Ryan Truscott
This species, which grows wild in the hot dry regions of Zimbabwe has unisexual flowers arranged on spikes: male flowers at the top, female flowers at the base.
But it is the fruit that is probably the tree’s most eye-catching feature.

Pawnbroker capsule. Photo: Ryan Truscott
First there is that three-lobed fruit capsule. It is delicate and brown with thin walls that are easy to scrunch between your fingers.
Inside are round seeds that look just like chocolate Malteasers. They are fused together in such a way that they resemble the three gold spheres traditionally hung from a rod outside a pawnbroker’s shop.
For South Africa-based writer Mary Armour, who grew up on forestry estates in eastern Zimbabwe when her father worked for the Forestry Commission, the Pawnbroker tree evokes memories of home.

Pawnbroker seeds. Photo: Ryan Truscott
“I first encountered Excoecaria bussei in a thicket near a dried-up riverbed near Shurugwi where my uncle farmed,” she told me via email this month.
She recalls how a family friend, who identified the tree by another of its common names — the Pepper-seed tree — once gave her a seed to taste.
“It was almost tasteless to start with; dry and musty, then had an after-taste of peppery burning, unpleasant and probably toxic.”
Her aunt would sometimes pick and dry the fruit for potpourri.
Mary, who has a keen interest in the lives of many of the early European plant collectors in Africa, said that the tree gets its specific name from explorer Walter Carl Otto Busse.
Busse was a German agricultural officer in Tanzania (then Tanganyika) at the turn of the century.

Pawnbroker Leaves. Photo: Ryan Truscott
“In June 1900, Busse spent five months travelling in east central Tanganyika where he collected some 400 plants, one of them subsequently classified as E. bussei,” she said.
Busse later returned to Germany and died in 1933.
Mary remembers one afternoon walking on the farm in Shurugwi when she found a Pawnbroker tree surrounded by fluttering hordes of brown butterflies.
“I thought at first they were moths because of their wispy insignificant appearance,” she says.
They were later identified as Morant’s tree nymphs, Sevenia morantii. Their caterpillars are known to feed on the leaves of E. bussei.
And the leaves are certainly striking: dark green, glossy with serrated margins.
The first time Mary caught sight of the leaves they stood out against the dryness of the surrounding bush. She picked one to reveal the milky white latex that, being from the Euphorbiaceae family, is a key feature of this tree.
Wild animals are believed to avoid the tree because of it.
It makes sense that in the dry season, when you’re the only thing that’s green, you’ll need protection against animals that want to eat you.
-Ryan Truscott
Continued from Tree Life 485, January 2021
Epiphytic Orchids in Zimbabwe (2) Epiphytes and their Host Trees
The first impression, when looking at the distribution of epiphytic orchids within a forest, is the lack of order. It seems that they are able to grow anywhere on the trees. Distribution patterns only emerge when a substantial number of trees are analysed, if possible from the base of the stem up to the outer branches in the canopy.
Microclimatic Requirements
Most orchid species are more or less restricted to one or two particular sections of the host tree, as shown in the Figure below. Only a few species, the so-called generalisers, are able to colonise a number of sections more evenly. Johannson (1974: 69), who did some ecological orchid research in Liberia, West Africa, found that approximately 20% of orchid species grow mainly on the stem while 80% are found in the canopy. But the favourite areas of the canopy are the lower and middle parts of the larger branches. These different locations within the tree reflect on the microclimatic requirements of the respective orchid species. For example, species growing on the stem require more shade, while those in the outer canopy receive plenty of light. On the other hand, species growing in the inner canopy are partly shaded.
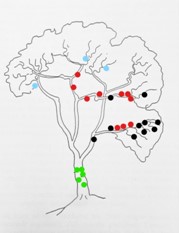 An example for the distribution of epiphytic orchid species on a tree, from Morris (1970: fig. 2).
An example for the distribution of epiphytic orchid species on a tree, from Morris (1970: fig. 2).
Explanation: ● Angraecopsis parviflora, ● Polystachya vaginata, ● Bulbophyllum maximum, ● Cyrtorchis arcuata.
The Orchid – Host Relationship
A question which is often asked is: On which trees do epiphytic orchids grow? Are there any preferences? And if yes: why? Summarising it can be said: Every species has developed its own preference, but there are tendencies. Unfortunately, there is not much information on the subject.
Brian Morris (1970: 5), the pioneer orchid collector in neighbouring Malawi, was quite interested in this question. He observed for example “that there are few specific host/orchid relationships and the majority of orchids are found on a wide variety of trees with little or no selective basis, (…) what is apparent is the tendency for many trees to be, what I have termed, ‘orchid-prone’. Parinari mbola and Uapaca kirkiana in lowland savannah; Brachystegia spiciformis on the higher slopes; Syzygium cordatum and Bridelia micrantha in gallery forest; Ilex mitis and Podocarpus milanjianus [now Podocarpus latifolius] in montane forest; all these are clearly ‘orchid-prone’ trees, sometimes strikingly so. Thus 71% *) of the epiphytic orchids recorded by myself in the Blantyre-M[u]lanje districts were noted on five species of tree, and the physical and chemical properties that make such trees good hosts would be an interesting field of study.” From the Table below it can be said that there are tree species which are more suitable hosts than others. One species which is more suitable is obviously the Musasa, Brachystegia spiciformis, followed by Syzygium cordatum and Bridelia micrantha. Similar observations can be made in Zimbabwe, though precise statistics are not available.
(Footnote) *) In the Table below this percentage rises actually to 79% because the Table has been modified, i.e. tree and orchid species which do not occur in Zimbabwe have been omitted.
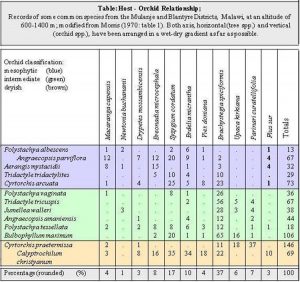
The Epiphytic Orchids of Malawi. The Society of Malawi
There are cases of more specialised relationships. Morris (1970: 5) reported for example from Malawi: “There are however a few orchids which would appear to associate with specific hosts. Bulbophyllum intertextum on Newtonia buchananii, Polystachya johnstonii on Vellozia splendens [now Xerophyta splendens] come to mind. Mention may be made of the fact that the genus Aerangis is almost restricted to smooth-barked trees.” On the other side there are species which are not specialised: “The common epiphyte Calyptrochilum christyanum for instance has been recorded on over 20 different trees.”
What makes one tree species more suitable for epiphytic orchids than others? Firstly, the properties of the bark come to mind. For example, Brachystegia spiciformis is a favourable host, Brachystegia glaucescens is not due to its smooth bark which partly peels off. No epiphytic orchids have been observed on trees with fire protection bark. But apart from the physical / mechanical properties of the bark, it is first of all the presence of certain fungi colonising the bark which qualifies a tree species to be suitable as a host. These fungi are required by the orchid seedlings as some kind of ‘nursing aid’ until they develop green leaves and can start producing their own food by photosynthesis. Obviously, fungi species are tree specific, some more than others. Orchid species only follow the fungi.
References
Johannson, D. (1974): Ecology of vascular epiphytes in West African rain forest. Acta Phytogeographica Suecica 59, Uppsala/Sweden
Morris, B. (1970): The Epiphytic Orchids of Malawi. The Society of Malawi
-Werner Fibeck
Tree walk at National Botanic Gardens 2 Jan 2021
First outing of the New Year brought 8 people to the Gardens. The weather was perfect with temperature, humidity and a light breeze as you would normally only dream of. The intermittent sunshine between white woolly clouds and the clear blue sky and dust free air after last night rains, made all the green come out in a crystal clear light.
Our Chairman was absent today, but the little group had enough enthusiasts to decide which way we were going to walk. From the car park we could see Combretum celastroides, Jesse-bush combretum, coming into flower. Meg made us attentive of the scales on the underside of the leaves. It is a low altitude tree from the Zambezi area.
Going along the paved path we passed Chionanthus battiscombei, Small-fruited ironwood with its glossy dark green leaves. The domatia visible on the leaves make it easier to identify it. We came to the Cola millenii, a Cola species not indigenous here. We always look at this beautiful small West African tree standing along the path. Someone spotted a flower in the tree and for the first time I could marvel at the small maroon brown flower about 1 cm wide. Then Jim spotted a second flower, still young and more of a pink-red hue.
With Mark Hyde in our midst, we not only learn about trees but also the names of weeds and grasses. A new little plant was a Crossandra species. It had a striking red flower and is from the Acanthaceae family that usually have attractive eye catching flowers.
 Passing by the Commiphora merkerii, Zebra-bark commiphora, Mark mentioned that it’s now renamed Commiphora viminea
Passing by the Commiphora merkerii, Zebra-bark commiphora, Mark mentioned that it’s now renamed Commiphora viminea
We then saw another beautiful red flower, better known to us as it is our National flower, the Flame lily. Its scientific name placing it at the top of the beauty list: Gloriosa superba. It was growing under a Hippocratea species, Paddle-pod.
Continuing our way, crossing the lawn, we came to the Monodora junodii, green-apple, that stands next to a Manilkara mochisia, lowveld milkberry. Like most Annonaceae, its leaves grow in one plane, also called distichous. Not far from this copse of trees there is a Galpinia transvaalica, Wild pride-of-India. In the hope of seeing its attractive panicles of flowers that are short lived, we went there but no flowers yet.

Disperos anthoceros. Photo: Werner Fibeck
Then Werner, our orchid specialist, diverted our attention to a small 2-leaved young plant growing through leaf mould: Disperis anthoceros, a native ground Orchid, that is common in the Gardens here.
We went to see the flowering Acacia schweinfurthii . Now Senegalia schweinfurthii, with globular flowers unusual for this genus with the other species (Vachellia) having inflorescences in spikes.
Mark looked at another weed: Bidens bipinnata, Spanish Black Jack which has fine lanceolate bipinnatifid leaves, quite different from Bidens pilosa, Black Jack. It has yellow ray flowers.
Another weed which is quiet common and has very attractive blue flowers was spotted and named Solanum seaforthianum, Brazilian nightshade. A common creeper or climber once introduced as a garden plant and now common in the tropics.
We then arrived at the Rhigozum brevispinosum, Simple leaved rhigozum. Too late again to see the beautiful flowers of this small tree with simple and rarely 3-foliate leaves.

Rhigozum brevispinosum. Photo: Jan Van Bel

Rhigozum obovatum. Photo: Jan van Bel
A few meters away there is the Rhigozum obovatum, Yellow pomegranate. This Bignoniaceae is usually 3-foliate with few single leaves. We again missed the flowers as was visible from the new pods that were already mature. Remarkable is the pod that opens in the middle of its broad sides.
A Jasminum fluminense was weaving its way through this shrub-like tree.
It was time to start going back. On the way we were talking about the next outing. Little did we know that another one month Covid 19 lockdown had just been announced. So for a month we will be creeping through our home gardens looking for weeds. Thanks to Mark’s information on weeds and small plants we can now divert our attention to from the trees we have been watching for years and are now too familiar with. With the abundance of rain we had, plenty of green to look at and trying to name it.
-Jan Van Bel.
INSECT-PLANT RELATIONSHIPS
TREE LIFE No.153 (November 1992) contained a very interesting article by Rob Paré on the close relationships between insects and plants. This is worth reproducing in full: Ed.
Virtually every species of butterfly or moth, worldwide, depends on the leaves of one plant or another as food for its developing larvae. A few species utilize either flowers, fruits, or seeds, some bore into the stems, some exist on various algae, and some are even carnivorous – but that is a subject all on its own.
There is virtually no plant anywhere that doesn’t bear evidence of having had part of it eaten by some insect or other. There are many recorded relationships between plants and insects, but those we know nothing about must run into millions. I have been studying the life histories of the Zimbabwean butterflies for several years, so my observations are restricted to that field.

Vanessa cardui. Photo: Wikipedia
Some of our butterfly species are dependent on a single plant or tree species for their larval food, while others are liable to utilize two or more species. It is very significant that where additional plant species are used, they are usually quite closely related – same genus or same family. Certain butterfly species are able to utilize a very wide range of plants. The Painted Lady (Vanessa cardui) is a good example of this ability, and as a result it is found almost anywhere in the world where there are butterflies. At the other extreme, some butterfly species occupy very restricted ranges, sometimes as little as one or two hectares, although to be fair, climatic and geographic factors are often part of the cause. The presence of a particular butterfly species in an area is usually an indication that its foodplant also occurs there. The converse doesn’t necessarily apply, as climatic factors sometimes restrict [a butterfly’s] range in spite of wide-ranging foodplants. A good example of this is the beautiful Flame-bordered Charaxes, Charaxes protoclea azota, which uses both Brachystegia spiciformis (musasa) and Afzelia quanzensis (pod mahogany), and yet is restricted to a fairly narrow strip along the moister (?) Eastern Highlands. Another Charaxes species, C. guderiana, turns up fairly often along the Zambezi River around Chirundu, at least 30 kilometres from the nearest Brachystegia and Julbernardia on the Escarpment, which are its known foodplants. Occasional individuals could certainly fly that distance, but the regularity of its occurrence, and the fresh condition of specimens taken at the river, indicate that it is using an, as yet, unknown tree there. I’m assuming it will be a tree, as the Charaxes, almost without exception, use trees or their saplings. Knowing that, it will most likely be a tree fairly close to the known ones. Tamarindus indica (tamarind) is my guess, or possibly Cordyla africana (wild mango). I have both of these growing at home, so it will be a fairly simple matter to trap a live female, and sleeve-net her on a spray of fresh foliage, where she will lay her eggs if the leaves give her the correct signals. If not, we scratch our heads and try again – Dalbergia or maybe Xanthocercis?
This brings us to the really interesting bit. Leaves of almost any plant, anywhere in the world, contain all the nutrients that any caterpillar needs for growth. The implications of that fact are potentially catastrophic for this planet – all plant life would have been guzzled out of existence long ago if it were not for the control mechanisms built into insects. Let’s examine briefly how a female butterfly locates the foodplant in order to lay her eggs. Firstly, the air temperature must be at a reasonable level – at least 25 degrees C. The female first detects the presence of the required plant with her antennae, picking up the minute traces of volatile `essential oils’, as they are known, given off by the plant into the surrounding air. Once she has located it, she flies through and around the foliage, alighting every so often to check the suitability of the leaf via special sensory organs in her feet, known as chemoreceptors, which must give the correct signals if she is to continue.
The age of the leaf is important here, as most species can only use fairly young to mature material.
Dust on the leaf surface will usually make the female reject the plant and move on, as will various repellant substances, like ethylene, which the plant emits when it is already being fed upon by caterpillars. Once the chemoreceptors in the feet, and others at the tip of the abdomen, have been satisfied, egg laying begins. Somehow the butterfly has gauged the size of the foodplant, and will lay only a few eggs on it before moving on to seek out others. This dispersion seems to be beneficial to both parties, as the plant is not `overgrazed’, and the predators and parasites have a hard time finding the larvae. The abundance of any particular foodplant seems to have very little bearing on the population level of the butterfly species that feeds on it. Julbernardia globiflora is a good example. It is arguably the commonest and most widespread of all Zimbabwe’s trees, and is known to be the foodplant of at least 11 different butterfly species, none of which is particularly common. In all my wanderings in the bush, I have seldom come across larvae of more than one species on an individual J. globiflora. It seems to be a case of first-come-first-served! Once the eggs hatch the larvae usually eat their egg shells as their first meal before starting to eat the foliage, which must now pass a further series of chemoreceptors in and around the mouthparts. Firstly, small 3-segment antennae on either side of the jaws must satisfy the larva that the food is the correct one. Then, tiny sensory palps must also be satisfied. Interestingly, the surgical removal of these palps has resulted in the larva happily eating almost any type of leaf material offered. As if that were not enough, a final set of chemoreceptors is [located] at the beginning of the digestive tract, and these cause a violent reaction if offended, causing the larva to vomit up all it has eaten.
In the wild the larval chemoreceptors are largely a formality, but when one comes to introduced plants of similar genera to local ones, although the female may be fooled by them, the larvae seldom are.
I have done some interesting experimentation where, although a female cannot be induced to lay on a plant closely related to her usual foodplant, her offspring, transferred as eggs, have no choice but to eat the new plant, and it often passes their test, even having failed their mother’s!
While searching patiently a few years ago for larvae of the, then, unknown Charaxes chittyi (recently named after the late JCO Chitty, of Harare), I noticed something very interesting. I had seen females of that species showing more than passing interest in Brachystegia boehmii (mupfuti), and decided that that must be its foodplant. In an area where at least 50% of the trees were B. boehmii, it was a long, drawn-out search, eventually rewarded by the discovery of a few larvae that did finally turn out to be C. chittyi. During the search I had noticed that the very occasional tree had smooth (glabrous) leaves instead of the usual hairy ones, and it eventually dawned that larvae had been found only on these glabrous ones. Thereafter, the search became easy, as I only needed to feel the leaves to know whether to search or pass on to the next tree. I tried to feed hairy leaves to some of the larvae, and they reacted so violently that I might as well have fed them with DDT! It was lethal to them. Can this just be the hairs, or is there a significant chemical difference in the leaves? I have been able to force females to lay their eggs on the hairy leaves, but the larvae were unable to eat them. I have noticed a similar situation with a different butterfly species on Dichrostachys cinerea (chinese lantern, or sickle bush), where the subspecies nyassana, with its hairless leaves, supports larvae, while ssp africana cannot; and yet another on Ficus sur (Cape fig), where only the occasional glabrous specimen will support larvae of the Map butterfly (Cyrestis pantheus), whose life history has only recently been discovered.
As an interesting aside, in April 1992 I took a walk in the Fuller Forest, just south of Victoria Falls, and found that the Brachystegia boehmii there are, almost without exception, all glabrous-leaved. I don’t think Charaxes chittyi has discovered this situation yet! Unfortunately, it was the wrong time of year to collect seed; those that I have carefully collected at Bindura off glabrous trees, and germinated, have produced hairy seedlings, much to my frustration!
One final interesting observation, by no means exhaustively researched, but nonetheless significant, is the fact that of the 85 families of the National Tree List (Drummond 1981), only 31 contain species that are known to be foodplants of butterfly species. Many life histories are yet to be discovered, so there is an incredible amount of hands-on, eyes-on work to be done. Worldwide literature is full of clues for us – for example, the Map butterfly, just mentioned, has related species in South America and the Far East, but no relatives in Africa. Those relatives whose life histories are known are dependent on various Moraceae, which give the clue to where to start looking here. Examining saplings of Trilepisium madagascariensis in Chirinda Forest led to the discovery of very strange larvae, which turned out to be those of Cyrestis pantheus – a great thrill. Since this species occasionally turns up hundreds of kilometres away from its `home’ habitats in the eastern districts forests, we assumed it would also be using a Ficus species, and F. sur proved to be the one – although only the glabrous individuals. The possibility that other Ficus species are also used is very real and only time and search will reveal it – and such discoveries are their own reward.
[Comment 2000: According to Richard Cooper (1973), Butterflies of Rhodesia (Longman’s Bundu Series), Charaxes protoclea azota is known only from the Burma Valley and Mount Selinda areas in Zimbabwe, but is common in parts of Mozambique. Mean annual rainfall in the Zimbabwe part of its range would be around 1400-1500 mm, and this could not really be described as the “moister” Eastern Highlands.Rob Paré’s discovery that the larvae of Charaxes chittyi will not touch Brachystegia boehmii that have hairy leaves is extremely interesting, and it makes one wonder whether it is the hairs, per se, that make the leaves unpalatable, or some chemical within the hairs, or in hairy leaves generally. “The Book” (Coates Palgrave) says that the upper surface of mature leaves of B. boehmii are without hairs, but that all other parts are finely hairy; Rob Paré’s observations indicate that this description needs revision. Similar observations were made on glabrous and hairy leaves of other tree species with other butterflies, which brings up the question of chemical analyses of hairy and glabrous leaves within a tree species. Chemotaxonomy has been used as a tool in the classifications of Pinus and Eucalyptus, and it would be interesting to see whether it could be applied in some of our own genera. Insect feeding preferences associated with apparently minor morphological differences within a plant species suggest that there may be scope for research into this phenomenon.
Rob Paré’s frustration at finding only hairy seedlings germinating from seed collected from glabrous B. boehmii is understandable, but this is quite common in other species. However, the hairy, juvenile leaves of seedlings give way, in due course, to glabrous leaves in the older plant. It would be interesting to know if this is happening in his mupfuti.
As a follow-up to his article on Plant-Insect Relationships, Rob Paré hosted a Tree Society outing to his farm near Bindura on 15 November 1992.
Rob Pare
UPDATE ON THE TREE SOCIETY OF ZIMBABWE WEBSITE – 24th Jan 2021
We had a drop in hits over the festive period but the numbers appear to have come up since then.
Total hits for the last 30 days ending 24th January 2021 were 1081 with almost half of them coming from Zimbabwe as expected – breakdown: Zimbabwe 530, South Africa 158, United Kingdom 128, United States 59, Australia 59, China 18, Canada 11, India 10, Germany 9 and New Zealand 9 plus hits from another 47 countries.
Some facts:
More males than females visit our site 54.15% / 45.85%
Age groups 18 – 34 years constitute 61% of the hits whilst ages 65+ only account for 5.5% which definitely does not reflect our membership!
A few months ago we had almost 1500 hits per month so interest has been tailing off or because of the second wave pandemic lockdown perhaps there is less computer accessibility.
In the last year 76% of the hits came via Google.
The all time high was 80 hits in one day on the 1st October 2020.
In the last month, the highest was 55 hits in one day – 11th January 2021
-Tony Alegria
TONY ALEGRIA CHAIRMAN